Tirzepatide Complete Guide — 2025 Edition

[Quick Guide] Tirzepatide Price & Where to Buy (Updated for 2025)
If you are searching for how much tirzepatide costs and where to buy tirzepatide legally, safely, and at a more affordable price, here is the fastest overview:
- U.S. originator drug: US$399–499 per month (single-dose vial version), available via the manufacturer’s official LillyDirect channel + telehealth platforms, where you obtain an online prescription and have the medicine shipped to your home.
- Taiwan originator drug: about NT$12,000–18,000 per month; you must obtain a prescription from a physician and it cannot be purchased online.
- China originator drug: about CNY 1,200–1,700 per month; you can obtain a prescription via “online consultation” services on JD.com / Taobao and have it delivered afterwards.
- Cross-border generics: the price is less than half that of the originator drug. Under each country’s personal-use regulations, you may legally purchase a small quantity for self-use (typically up to 3 months), with worldwide shipping available.
Therefore, if your goal is to find where to buy tirzepatide at the lowest price and with the most convenience:
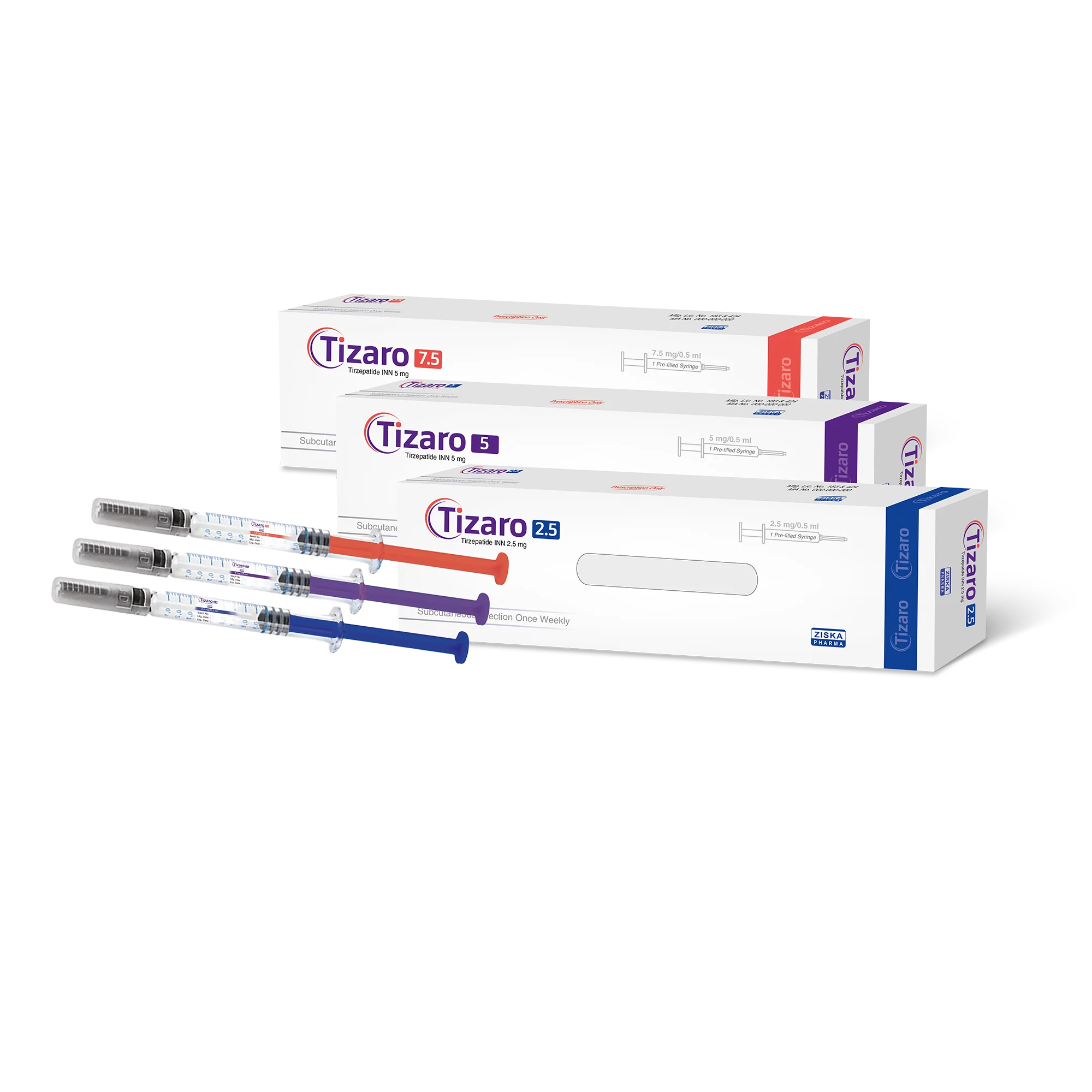
cross-border purchase of Bangladesh DGDA-approved tirzepatide generics (for example, Ziska Pharma’s Tizaro) is currently the best option in terms of both price and convenience.
1. Tirzepatide price: originator drug price comparison by country
Tirzepatide was approved by the U.S. FDA in November 2023 for chronic weight management, which sparked a worldwide weight-loss boom. Many celebrities (such as Tesla CEO Elon Musk, actress Whoopi Goldberg, and singer-songwriter Meghan Trainor) have publicly acknowledged using GLP-1-class injections to control their weight.

Celebrity buzz has driven many consumers to take a strong interest in tirzepatide. This article walks you through reference retail prices for tirzepatide across major markets and helps you choose legal and more affordable purchase channels.
Below are the monthly out-of-pocket reference prices for originator tirzepatide in each market.
Out-of-pocket tirzepatide prices (converted into U.S. dollars, USD)
| Dose (mg) | United States 🇺🇸 | China 🇨🇳 | Taiwan 🇹🇼 | Australia 🇦🇺 |
|---|---|---|---|---|
| 2.5 | $1,080 | $240 | $410 | $260 |
| 5 | $1,080 | $390 | $450 | $300 |
| 7.5 | $1,080 | $540 | $490 | $390 |
| 10 | $1,080 | $680 | $530 | $390 |
| 12.5 | $1,080 | $810 | $570 | $480 |
| 15 | $1,080 | $940 | $610 | $480 |
Out-of-pocket tirzepatide prices (converted into New Taiwan dollars, TWD)
| Dose (mg) | United States 🇺🇸 | China 🇨🇳 | Taiwan 🇹🇼 | Australia 🇦🇺 |
|---|---|---|---|---|
| 2.5 | $32,000 | $7,000 | $12,000 | $8,000 |
| 5 | $32,000 | $12,000 | $14,000 | $9,000 |
| 7.5 | $32,000 | $16,000 | $15,000 | $12,000 |
| 10 | $32,000 | $20,000 | $16,000 | $12,000 |
| 12.5 | $32,000 | $24,000 | $17,000 | $14,000 |
| 15 | $32,000 | $28,000 | $18,000 | $14,000 |
Out-of-pocket tirzepatide prices (converted into Chinese yuan, CNY)
| Dose (mg) | United States 🇺🇸 | China 🇨🇳 | Taiwan 🇹🇼 | Australia 🇦🇺 |
|---|---|---|---|---|
| 0.25 | ¥3590 | ¥1010 | ¥1730 | ¥1870 |
| 0.5 | ¥3590 | ¥1150 | ¥1940 | ¥2090 |
| 1.0 | ¥3590 | ¥1150 | ¥2160 | ¥2300 |
| 1.7 | ¥3590 | ¥1730 | ¥2810 | ¥2810 |
| 2.4 | ¥3590 | ¥1730 | ¥3460 | ¥3530 |
As you can see, tirzepatide is most expensive in the United States, where the monthly cost exceeds US$1,000. However, Eli Lilly provides single-dose vial tirzepatide products through the LillyDirect channel, which can bring the monthly cost down to US$399–499.
In the Asia-Pacific region (including Taiwan, China, Australia, etc.), tirzepatide prices are generally lower than in the U.S. However, because weight-loss use is typically not covered by health insurance at present,it still represents a high out-of-pocket expense and can be a substantial burden for long-term treatment.
2. Tirzepatide price: insurance coverage by country
To address the high cost of tirzepatide, the United States, the United Kingdom, and Japan provide some level of insurance coverage for tirzepatide used for obesity treatment, but eligibility criteria are generally strict.

🇺🇸 United States: partial private insurance coverage, but strict criteria
Some private insurance plans cover tirzepatide for weight-loss indications, but patients must meet BMI criteria and obtain prior authorization from a physician. Under certain plans, patients’ monthly out-of-pocket cost for tirzepatide can be as low as US$25. However, commercial insurance typically imposes very strict conditions and often limits treatment duration to only 1–3 months, which remains challenging for patients needing long-term therapy.
🇬🇧 United Kingdom: NHS rolling out phased access
The UK National Health Service (NHS) launched a large-scale rollout program in 2025, under which patients with severe obesity (BMI ≥ 40, or ≥ 35 with comorbidities) can be referred by general practitioners to receive tirzepatide injections free of charge. The program is expected to serve 220,000 patients over three years.
🇯🇵 Japan: partial coverage under National Health Insurance (NHI)
Starting from February 2024, Japan included tirzepatide in its National Health Insurance (NHI) scheme for the treatment of obesity-related diseases. For eligible patients, the out-of-pocket tirzepatide price is almost negligible, but coverage is restricted to strict criteria (BMI ≥ 27 with at least two obesity-related comorbidities, or BMI ≥ 35 with at least one comorbidity).
3. Where to buy tirzepatide at the best value? Originator vs generics

What is a generic drug, and why are generics cheaper?
A generic drug is a medication that contains the same active ingredient, dosage, route of administration, and therapeutic effect as the originator (brand-name) drug, but is not manufactured by the original innovator company. Generic drugs are usually priced lower than originator products, helping to reduce healthcare costs and providing economic benefits for both patients and health systems.
In theory, there should be no tirzepatide generics before 2036.
To protect innovator companies’ interests, U.S. patent rules grant new drugs at least 12 years of market exclusivity after approval. Patent protection related to tirzepatide products will not expire until as early as 2036, which means no other company can manufacture originator-equivalent brand-name products with the same active ingredient before then.
However, Bangladeshi manufacturers are permitted to produce tirzepatide generics.
Bangladesh is one of the world’s largest producers of generic medicines. Under exemptions granted by the World Trade Organization (WTO) for least-developed countries (LDCs), local manufacturers are allowed to produce generic versions of patented medicines that are still under patent protection elsewhere.
Leading Bangladeshi pharmaceutical companies maintain strict quality standards for their generic medicines:
- They hold WHO-recognized quality certifications (such as WHO-PQ, GMP).
- They are formally approved for marketing by the Directorate General of Drug Administration (DGDA) in Bangladesh.
- They have passed bioequivalence (BE) studies, demonstrating equivalent efficacy to the originator drug.
For tirzepatide generics manufactured in Bangladesh, the tirzepatide price is typically only half—or even less—of the originator price.
These generics are often shipped directly from the manufacturer, without multiple layers of distribution, making pricing transparent and reasonable, and providing a reliable and affordable option for self-pay patients.
The world’s first tirzepatide generic was launched by long-established Bangladeshi manufacturer Ziska Pharmaceuticals, under the brand name Tizaro. Ziska has already produced more than 100,000 doses of tirzepatide generics, primarily serving patients in Bangladesh and neighboring Asian countries.
4. Where to buy tirzepatide? Originator drug purchase channels
1. Offline hospitals and clinics
Tirzepatide is a prescription medication that must be prescribed by a licensed physician. It is usually prescribed after an evaluation in endocrinology, metabolism/diabetes clinics, or weight-management/aesthetic clinics. For weight-loss indications, patients must meet the physician’s clinical criteria (such as elevated BMI and comorbidities), and treatment response needs to be monitored.
2. Telehealth platforms
In a small number of countries with well-developed telehealth systems, consumers can obtain a tirzepatide prescription for weight management through online consultations and then have the medication delivered by partner pharmacies.
🇺🇸 United States – where to buy tirzepatide: LillyDirect
In the U.S., Eli Lilly has launched the official platform LillyDirect, which provides home delivery of tirzepatide. However, LillyDirect itself does not provide medical consultations. Patients must first receive a prescription through partner telehealth providers and can then arrange for medication delivery via LillyDirect.
🇨🇳 China – where to buy tirzepatide: Taobao, JD.com
Patients in China can obtain prescriptions via health-service portals on the major e-commerce platforms Taobao and JD.com, and partner pharmacies will ship the medication directly. Note that Taobao and JD.com do not support cross-border delivery; recipients must be located within mainland China.
Please note that in many Asian countries (such as Japan, South Korea, Taiwan, etc.), regulations do not allow patients to obtain tirzepatide weight-loss prescriptions via telehealth platforms.5. Where to buy tirzepatide? Generic-drug purchase channels
1. Cross-border medication platforms
Legally manufactured generics can be purchased through cross-border medication platforms (such as Mediva Rx), which place orders directly with Bangladeshi generic manufacturers. The medicine is shipped directly from the manufacturer by air and typically arrives in about one week.
Many Asian countries (including Japan, Taiwan, China, Australia, etc.) allow individuals to import a small quantity of prescription medications from overseas for personal use, usually limited to no more than around three months of therapy per shipment. Resale or redistribution, however, is prohibited and may constitute a legal violation.
When purchasing small personal-use quantities, customs authorities in many countries typically do not require patients to submit prescriptions or diagnostic documents, nor do they levy customs duties on personal-use medicines. Regulations on personal cross-border medication purchases differ by country. For details, please refer to the Personal Import Guide for Self-Use Medicines.
When choosing generic medicines, always ensure that the products come from manufacturers with internationally recognized certifications and that the dosage matches the originator drug to safeguard efficacy and safety.
2. Pharmacies in Bangladesh
Some consumers choose to travel to Bangladesh and purchase medications directly at local pharmacies, then bring them back home. However, this approach involves higher time and travel costs and is not practical for everyone.
3. Private “daigou” resellers (not recommended)
At present, many private resellers purchase large volumes of generic medicines from pharmacies in Bangladesh and resell them in their home countries. Such practices may violate local regulations and expose consumers to risks of expired or counterfeit medicines.
More importantly, tirzepatide requires continuous cold-chain storage. If it is kept at room temperature for extended periods, its efficacy may drop or the product may degrade. The temperature control and storage conditions used by private resellers are often impossible to verify, so consumers should be extremely cautious.
6. Tirzepatide vs semaglutide: efficacy and price comparison
The two most popular injectable weight-loss medications at the moment are tirzepatide and semaglutide. Both belong to the GLP-1 drug class, but tirzepatide delivers more pronounced effects, especially for weight reduction.
According to the latest data from the SURMOUNT-5 trial:
- Patients on tirzepatide lost an average of about 20.2% of body weight, roughly 22.8 kg.
- Patients on semaglutide lost an average of about 13.7% of body weight, roughly 15.0 kg.
- In other words, on average, tirzepatide provided an additional ~6.5% body-weight reduction compared with semaglutide.

Why is tirzepatide more effective?
This is because tirzepatide uses a dual-target mechanism: it mimics both GLP-1 and another incretin hormone, GIP. This “dual agonist” design further enhances insulin secretion, prolongs satiety, reduces food intake, and improves fat-metabolism efficiency, resulting in a more comprehensive effect than drugs that act solely on GLP-1.
The table below summarizes the differences between the two GLP-1 products
| Item | Tirzepatide | Semaglutide |
|---|---|---|
| Brand name | Mounjaro | Wegovy |
| Targets | Dual GIP + GLP-1 receptors | GLP-1 receptor only |
| Manufacturer | Eli Lilly | Novo Nordisk |
| Dosing frequency | Subcutaneous injection once weekly | Subcutaneous injection once weekly |
| Weight-loss effect at 68 weeks | ~20.2% reduction | ~13.7% reduction |
| Uninsured monthly cost (U.S.) | ~US$1,080 | ~US$1,350 |
| Self-pay discount (U.S.) | US$399–499 (LillyDirect) | US$499 (NovoCare) |
From clinical data, tirzepatide delivers better weight-loss results than semaglutide, while their prices are not significantly different.
According to official figures released by Eli Lilly in August 2025, tirzepatide has already captured 57% market share in the U.S. (across both obesity and diabetes indications), surpassing semaglutide. This shows that consumers tend to choose tirzepatide, which offers better weight-loss outcomes at a similar price level.
7. What is “compounded tirzepatide,” and why you should avoid it now
Between 2023 and 2024, due to Eli Lilly’s supply shortages, tirzepatide experienced a severe global shortage. During this period, the U.S. market saw the rise of so-called “compounded tirzepatide”: products prepared by compounding pharmacies using similar raw materials based on prescriptions. These products were typically much cheaper than the originator drug and highly attractive to consumers. Under U.S. regulations, when a medicine is placed on the official “shortage list,” the FDA may temporarily allow qualified pharmacies (such as 503A pharmacies and 503B outsourcing facilities) to compound versions of that medicine to bridge the supply gap. At that time, many telehealth platforms and pharmacies offered compounded tirzepatide for self-pay use.
However, these compounded preparations were not authorized by the originator company and did not go through the full FDA approval process, lacking guarantees on quality, dose consistency, and sterile manufacturing conditions. The FDA has also warned that some compounded products may not contain the correct active ingredient at all and may even contain impurities or counterfeit substances.
By 2025, as Eli Lilly’s supply stabilized, the FDA removed tirzepatide products from the shortage list and formally prohibited all pharmacies from compounding or selling tirzepatide products. Pharmacies and clinics that previously sold such compounded drugs were ordered to discontinue them; violators may face enforcement actions and civil lawsuits.

For consumers, this sends a clear message: there are currently no legally marketed compounded tirzepatide products. If you still see tirzepatide offered at prices far below the market level from questionable sources, it is highly likely to be an illegal product and may even pose serious health risks.
Popular FAQs
Q1: How does tirzepatide work?
After you eat, your intestines release a type of incretin hormone called GLP-1, which sends signals to the brain that you are full, stimulates insulin secretion to control blood glucose, and reduces appetite.
Tirzepatide is a weight-loss injection that mimics GLP-1 activity and was developed by global pharmaceutical company Eli Lilly. In simple terms, tirzepatide prolongs satiety, suppresses appetite, and stabilizes blood sugar. It is a highly effective, medically supervised weight-management tool.

Tirzepatide is a once-weekly subcutaneous injection, originally developed to treat type 2 diabetes. Its pronounced weight-loss effect was later recognized, and regulatory agencies in multiple countries have approved it for obesity and overweight patients.
Countries and regions where tirzepatide has been approved include:
- 🇺🇸 United States: approved for type 2 diabetes and chronic weight-management indications.
- 🇪🇺 European Union / 🇬🇧 United Kingdom: approved for diabetes and weight-management uses.
- 🇹🇼 Taiwan: officially approved for weight-loss use in June 2025
- 🇯🇵 Japan: covered for diabetes under NHI; self-pay use for weight management is common.
- 🇨🇳 China: some hospitals in certain regions have launched obesity-treatment applications.
- 🇦🇺 Australia: approved for type 2 diabetes and chronic obesity management.
Q2: Why is tirzepatide so expensive?
Tirzepatide is expensive mainly because it is an innovative dual-target drug developed by Eli Lilly. The R&D process—from molecular design through large-scale clinical trials—costs several billion U.S. dollars, and there are currently few direct competitors. Before the patents expire, the manufacturer has strong incentives to adopt premium pricing to recoup investment. In addition, demand for obesity and diabetes treatments is high, while production capacity remains tight, further pushing up end-user prices.
Q3: Will tirzepatide prices come down in the future?
In the short term, a dramatic price drop is unlikely for three main reasons: first, patents will remain in force until at least 2036, giving Eli Lilly effective monopoly pricing power; second, global demand is continuing to grow while supply remains relatively tight, and this supply-demand imbalance supports high prices; third, the manufacturer is pursuing value-based pricing and is unlikely to voluntarily cut originator prices significantly.
However, prices may soften over the longer term if competing new drugs (for example, next-generation GLP-1/GIP combinations or oral formulations) enter the market, and if government and payer negotiations place greater pressure on pricing.
Q4: What is the relationship between tirzepatide and Mounjaro?
Mounjaro is the brand name under which Eli Lilly markets injectable products containing tirzepatide as the active ingredient worldwide, for the treatment of type 2 diabetes and obesity. “Tirzepatide” is the generic (INN) name of the molecule, while “Mounjaro” is the originator brand name.
Q5: Why isn’t there an oral tirzepatide?
Tirzepatide is a large peptide molecule. If taken orally, it would be broken down in the gastrointestinal tract and could not efficiently reach therapeutic concentrations in the bloodstream. Therefore, it must be administered via subcutaneous injection. To develop an oral tirzepatide formulation, challenges in stability, absorption, and dose volume must be overcome. Eli Lilly has not yet announced any concrete development progress for an oral version, so only injectable formulations are expected in the near future.
Q6: How do you inject tirzepatide? How often? Does it hurt?
Tirzepatide is injected subcutaneously once a week. It is recommended to inject into the subcutaneous fat of the abdomen, thigh, or upper arm, at a fixed weekly time, rotating injection sites each time. The needle is very fine, and most users report little to no significant pain during injection.
Q7: Which has milder side effects, tirzepatide or semaglutide?
The most common side effects of both drugs are gastrointestinal symptoms such as nausea, bloating, and constipation, especially during the initial period or when titrating doses. For most people, these symptoms gradually improve over a few weeks. Direct head-to-head data on side-effect severity vary across studies, and individual responses also differ. In general, carefully following the physician’s titration schedule, eating smaller meals, and avoiding high-fat or heavy foods can help reduce discomfort.
Q8: Will tirzepatide generics be less effective?
No. As long as the generic has passed international bioequivalence (BE) studies and relevant certifications, its absorption and clinical effect are equivalent to the originator drug, with no compromise in quality or safety.
Q9: Which tirzepatide dose should I choose?
It is generally recommended to start from the lowest dose of 2.5 mg, monitor how your body responds, and then titrate up stepwise to 5, 7.5, 10 mg or even 15 mg under your doctor’s guidance. Not everyone needs a high dose; the treatment regimen should be individualized by your physician.
Q10: Will my weight rebound after stopping tirzepatide?
If you stop treatment and do not maintain healthy eating and lifestyle habits, your weight may rebound. Therefore, whether you are using the originator drug or a generic, long-term weight management still relies on sustained diet control and regular exercise.
If you have further questions about tirzepatide or generic-treatment options, you are welcome to contact the professional team at Mediva Rx. We provide safe, compliant, and affordable medication-access options to support you in taking the first step toward better health management.
Disclaimer: The information above is for reference only. Treatment decisions should follow your physician’s instructions and be based on the official prescribing information and the latest clinical evidence.
GLP-1 Weight Management
GLP-1 medicines are FDA-approved for type 2 diabetes and weight management.
The leading options are tirzepatide (Eli Lilly) and semaglutide (Novo Nordisk).
Mediva Rx provides equally effective, more affordable generic versions.
-
Generic Mounjaro
 USD
USD