Tirzepatide – Injection
-
Detail
Product Description:
Tirzepatide is the world’s first dual agonist that acts on both GIP and GLP-1 incretin receptors. It promotes insulin secretion, reduces glucagon levels, slows gastric emptying, and suppresses appetite. It effectively controls blood glucose in patients with type 2 diabetes and supports weight management. Clinical studies show an approximate 15–20.9% reduction in body weight.
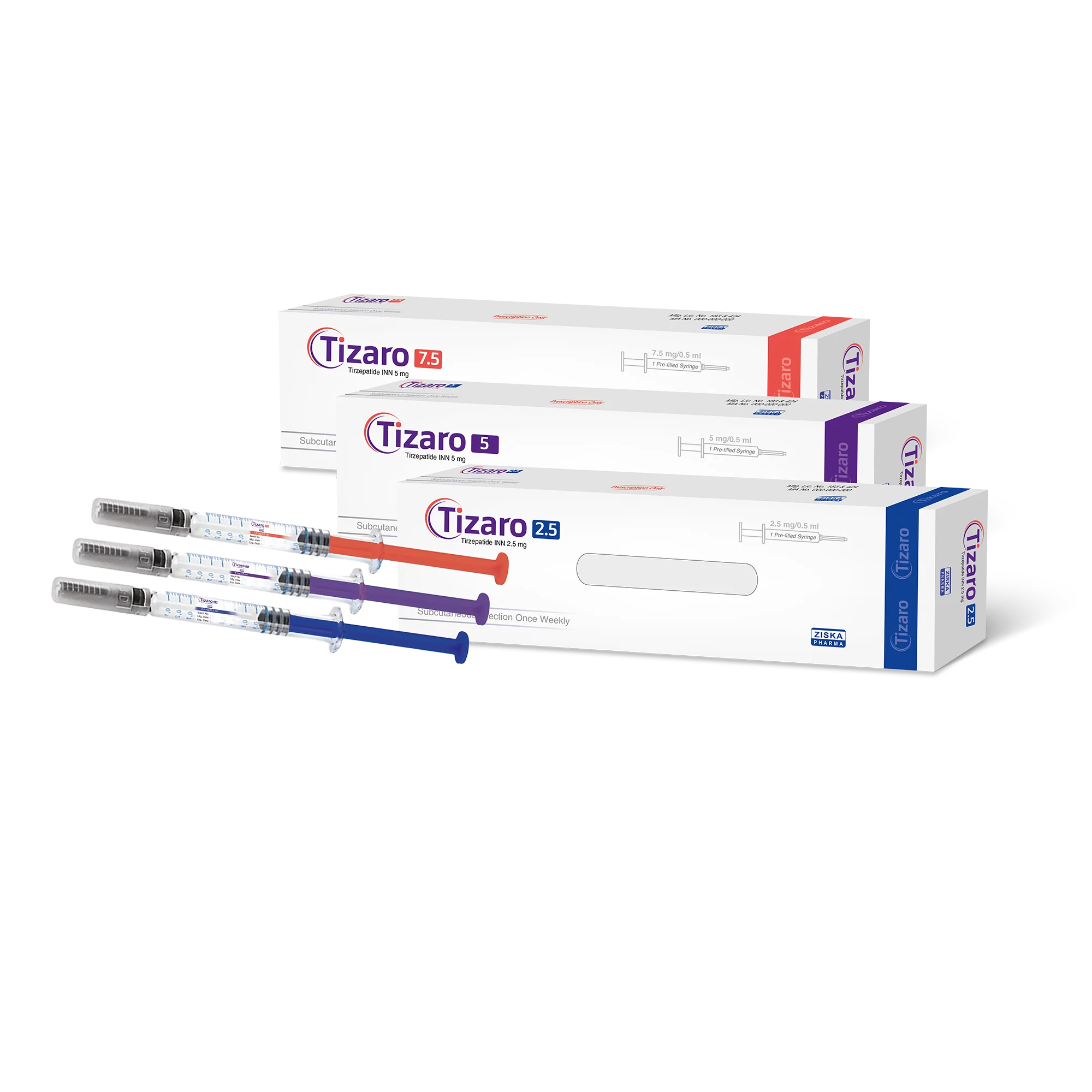
Active Ingredient: Tirzepatide
Dosage Form: Subcutaneous injection
Dosing Frequency: Once weekly. Each dosage pack includes 4 injections, enough for four weeks.
Brand Name: Mounjaro
Generic Name: Tizaro
Manufacturer (Generic): Ziska PharmaceuticalsIndications:
Tirzepatide is a dual agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. It is indicated for type 2 diabetes and obesity, serving as an adjunct to diet and exercise to improve glycemic control and support weight management.
Dosage and Administration:
Administer once weekly via subcutaneous injection. Tirzepatide is available in six strengths (2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg). Start with 2.5 mg weekly for the first 4 weeks. If well tolerated, increase sequentially to the next dose, maintaining each dose level for 4 weeks. The maximum dose is 15 mg weekly. If a dose is missed, administer within 96 hours; otherwise, skip the missed dose and resume the regular schedule. Injection sites include the abdomen, thigh, or upper arm subcutaneous tissue; rotate injection sites weekly.
Side Effects:
Common side effects include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, and abdominal pain. As with other GLP-1 medications, individual responses may vary.
Storage Conditions:
Store in a refrigerator at 2°C to 8°C. Do not freeze. Protect from light and keep out of reach of children.
Shipping Information:
Tirzepatide can remain at room temperature for up to 21 days. The product is shipped directly from the manufacturer and typically arrives within one week via air transport. Temporary exposure to room temperature during shipping does not affect efficacy.
Frequently Asked Questions:
Q: Is the tirzepatide injection sold by Mediva Rx the original brand?
A: No. Mediva Rx provides generic medication produced by one of Bangladesh’s top ten pharmaceutical companies. It contains the same active ingredient and offers equivalent therapeutic effect. It is tested by the Directorate General of Drug Administration (DGDA) and legally registered for sale.Q: Is the manufacturer of tirzepatide reliable?
A: Yes. Our partner manufacturer is Ziska Pharmaceuticals, one of the top ten pharmaceutical companies in Bangladesh. They possess R&D facilities, production lines, and quality-control laboratories, and meet international pharmaceutical manufacturing standards.Q: How do I use tirzepatide? How often is it injected? Does it hurt?
A: Tirzepatide is a subcutaneous injection administered once weekly. It is self-injected into the abdomen, thigh, or upper arm. Injection should be done at a consistent time each week, rotating sites regularly. Most patients experience little to no pain.Q: Which is more effective for weight loss, tirzepatide or semaglutide?
A: According to Eli Lilly’s SURMOUNT-5 Phase 3 clinical trial, tirzepatide demonstrated superior weight-loss outcomes compared to semaglutide. Over 72 weeks, tirzepatide achieved an average weight loss of 20.2% (about 22.8 kg), while semaglutide achieved 13.7% (about 15.0 kg).Q: Which has milder side effects, tirzepatide or semaglutide?
A: Both medications commonly cause gastrointestinal reactions such as nausea, bloating, diarrhea, and constipation, especially early in treatment or during dose escalation. Some patients report that tirzepatide feels slightly gentler in terms of side effects.Disclaimer:
This information is for reference only. Treatment decisions should follow physician guidance and the official prescribing information.
Prescribing Information:
-
Customer ReviewsNo comments
 USD
USD